Implementation details
This is what you need to consider in the implementation
How complex the transition from barcode to 2D code is depends largely on the individual circumstances, the objectives and the choice of product. For example, it is much easier to change the barcodes on a product where it is not integrated into the packaging layout but applied in the form of an adhesive label.
For a product like washing powder, where the EAN/UPC barcode has already been pre-printed on the empty packaging, the implementation is much more complicated. The only practical way would probably be to replace the barcode with GS1 DataMatrix codes that include a serial number in addition to the GS1 article number. In this way, the packaging can continue to be pre-printed, albeit with higher demands on the printing process and the filling process. In production, the packages are then filled as usual and the additional data is subsequently linked to the serial numbers in the ERP/PIM. However, this circumstance requires an exchange of master data at serial number level with the retail partners, who must then also be able to retrieve this data at all necessary points (such as the checkout environment).
Use our blueprints as a guide
In order to support you optimally during the implementation, we have developed blueprints for you. Please contact us if you need an additional blueprint.
| Blue Print |
is being or has been piloted at |
Complexity |
|
|---|---|---|---|
| Replace EAN/UPC barcode with GS1 DataMatrix |
|
low |
|
| Replace EAN/UPC barcode with GS1 DataMatrix for piece goods | Coop | medium | |
| Replace EAN/UPC barcode with GS1 DataMatrix for variable-quantity fresh products | GS1 Belgilux | medium | |
| Add EAN/UPC barcode with QR code and GS1 Digital Link | low | ||
| Supplement or replace EAN/UPC barcode or GS1 DataMatrix with QR Code and GS1 Digital Link | high | ||
| General serialization of products | high |
Blue Print: Replace EAN/UPC barcode with GS1 DataMatrix
Goals
- Reduction of the space required for the GS1 data carrier
- Use at the checkout (Point Of Sale) and in self-scanning
Steps
- Together with the supply chain partners involved in the process, determine which products are suitable for testing the transition.
- Define a schedule with your partners.
- Replace the EAN/UPC barcode with the GS1 DataMatrix code.
- Document your experience and the requirements that need to be met in your operation in order to start pilot projects with a trading partner.
Blue Print: Replace EAN/UPC barcode with GS1 DataMatrix for piece goods
Goals
- Add additional data such as MHD, batch number in the 2D code with the help of the GS1 Application Identifier (AI).
- Use at the checkout (Point Of Sale) and self-scanning
Steps
- Together with the supply chain partners involved in the process, determine which products are suitable for testing the conversion.
- Determine with your trading partner what additional data should be encoded in the 2D code in addition to the mandatory GS1 article number (GTIN).
- Consider the production process of the selected products and ensure that the additional data such as the BBD is available in the printing process.
- Contact your solution provider for the printing process and clarify whether the existing solution is capable of printing the necessary data in a GS1 DataMatrix.
- Check how you can cover the different requirements of your trading partners in your own production.
- Define a schedule with your partners.
- Replace the EAN/UPC barcode with the GS1 DataMatrix code.
- Document your experiences and the requirements that need to be met in your operation in order to start pilot projects with a trading partner.
Blue Print: Replace EAN/UPC barcode with GS1 DataMatrix for variable-quantity fresh products
Goals
- Use of the GS1 article number (GTIN) instead of the nationally unique GS1 article number (RCN) at best.
- Bar coding of product weight or individual price for quantity-variable fresh products
- Replacement of EAN/UPC barcodes by GS1 DataMatrix
- Using GS1 Application Identifier (AI) to add additional data such as MHD, batch number in 2D code
- Use at the checkout (Point Of Sale) and self-scanning
Steps
- Together with the supply chain partners involved in the process, determine which products are suitable for testing the conversion.
- Determine with your trading partner what additional data should be encoded in the 2D code in addition to the mandatory GS1 article number (GTIN).
- Consider the production process of the selected products and ensure that the additional data such as the BBD is available in the printing process.
- Contact your solution provider for the printing process and clarify whether the existing solution is capable of printing the necessary data in a GS1 DataMatrix.
- Check how you can cover the different requirements of your trading partners in your own production.
- Define a schedule with your partners.
- Replace the EAN/UPC barcode with the GS1 DataMatrix code.
- Document your experiences and the requirements that need to be met in your operation in order to start pilot projects with a trading partner.
Blue Print: Add QR Code and GS1 Digital Link to EAN/UPC Barcode
Goals
- Supplement EAN/UPC barcodes with QR codes that can be used for consumer engagement
- Add additional data such as MHD, batch number in the 2D code using the GS1 Digital Link standard
- Specify which web page is displayed if a consumer scans the QR code with their cell phone
Steps
- Together with the supply chain partners involved in the process, determine which products are suitable for testing the extension.
- Determine for your company what additional data should be encoded in the 2D code in addition to the mandatory GS1 article number (GTIN). This depends on the consumer needs you want to cover.
- Consider the production process of the selected products and make sure that the additional data is available in the printing process or can be pre-printed.
- Contact your solution provider for the printing process and clarify whether the existing solution is capable of printing the necessary data in a QR code. Take into account the space requirements.
- Add the QR code and GS1 Digital Link to the EAN/UPC barcode.
- Document your experience and the requirements that must be met in your operation in order to be able to start pilot projects with a trading partner.
Blue Print: supplement or replace EAN/UPC barcode or GS1 DataMatrix with QR code and GS1 Digital Link
Goals
- Add or replace EAN/UPC barcodes or GS1 DataMatrix with QR codes that can be used for consumer engagement
- Add additional data such as MHD, batch number in the 2D code using the GS1 Digital Link standard
- Determine which web page is displayed if a consumer scans the QR code with their cell phone
Steps
- Together with the supply chain partners involved in the process, determine which products are suitable for testing the extension.
- Determine for your company what additional data should be encoded in the 2D code in addition to the mandatory GS1 article number (GTIN). This depends on the consumer needs you want to cover and the requirements of your trading partners.
- Consider the production process of the selected products and ensure that the additional data is available or can be pre-printed in the printing process.
- Contact your solution provider for the printing process and clarify whether the existing solution is capable of printing the necessary data in a QR code.
- Replace the EAN/UPC barcode or GS1 DataMatrix with the QR code and GS1 Digital Link.
- Document your experience and the requirements that need to be met in your operation in order to start pilot projects with a trading partner.
Blue Print: General serialization of products
Goals
- Serialization of all products so that additional data can be exchanged with trading partners as consumer unit master data
- Most precise tracking and traceability
Steps
- Together with the supply chain partners involved in the process, determine which products are suitable for testing the extension.
- Determine for your company which and how the additional data should be exchanged as master data in addition to the serialized GS1 article number (SGTIN) encoded in the 2D code. This depends on the consumer needs you want to cover and the requirements of your trading partners.
- Consider the production process of the selected products and ensure that the additional data can be captured based on the serial numbers.
- Contact your solution provider for the printing process and master data exchange and clarify whether the existing solutions are capable of covering the necessary processes.
- Start serializing your products.
- Document your experiences and the requirements that must be met in your operation in order to start pilot projects with a trading partner.
2D Codes
The future of retail
One solution for the future is 2D-Codes, with which you can encode significantly more data than with the EAN/UPC barcode commonly used today.
What is a 2D-Code?
A 2D (two-dimensional) barcode is a graphic image that stores information not only horizontally like conventional linear (one-dimensional) barcodes, but also vertically.
2D codes, such as the GS1 DataMatrix or a QR code with GS1 Digital Link, require only a third of the space of a barcode and can contain much more information than an EAN/UPC barcode.
Will the barcode now be replaced?
At the 2021 General Assembly, GS1 Global decided that two-dimensional (2D) codes such as the GS1 DataMatrix or QR code with GS1 Digital Link may be used at the point of sale in retail from 2027.
The advantages of 2D-Codes
2D-supporters in Switzerland
EAN/UPC barcode and 2D-Codes in comparison
| EAN/UPC-Barcode |
GS1 DataMatrix |
QR Code with GS1 Digital Link | |
|---|---|---|---|
| Encoded GTIN |
[check-orange] |
[check-orange] |
[check-orange] |
| Low space requirement | [check-orange] | [check-orange] | |
| Additional data can be encoded | [check-orange] | [check-orange] | |
| BBD control at the checkout | [check-orange] | [check-orange] | |
| Enables the use of the GTIN for variable-measure consumer units | [check-orange] | [check-orange] | |
| Additional information on allergens, ingredients, product videos, etc. | [check-orange] |
Statements of 2D-Codes supporter
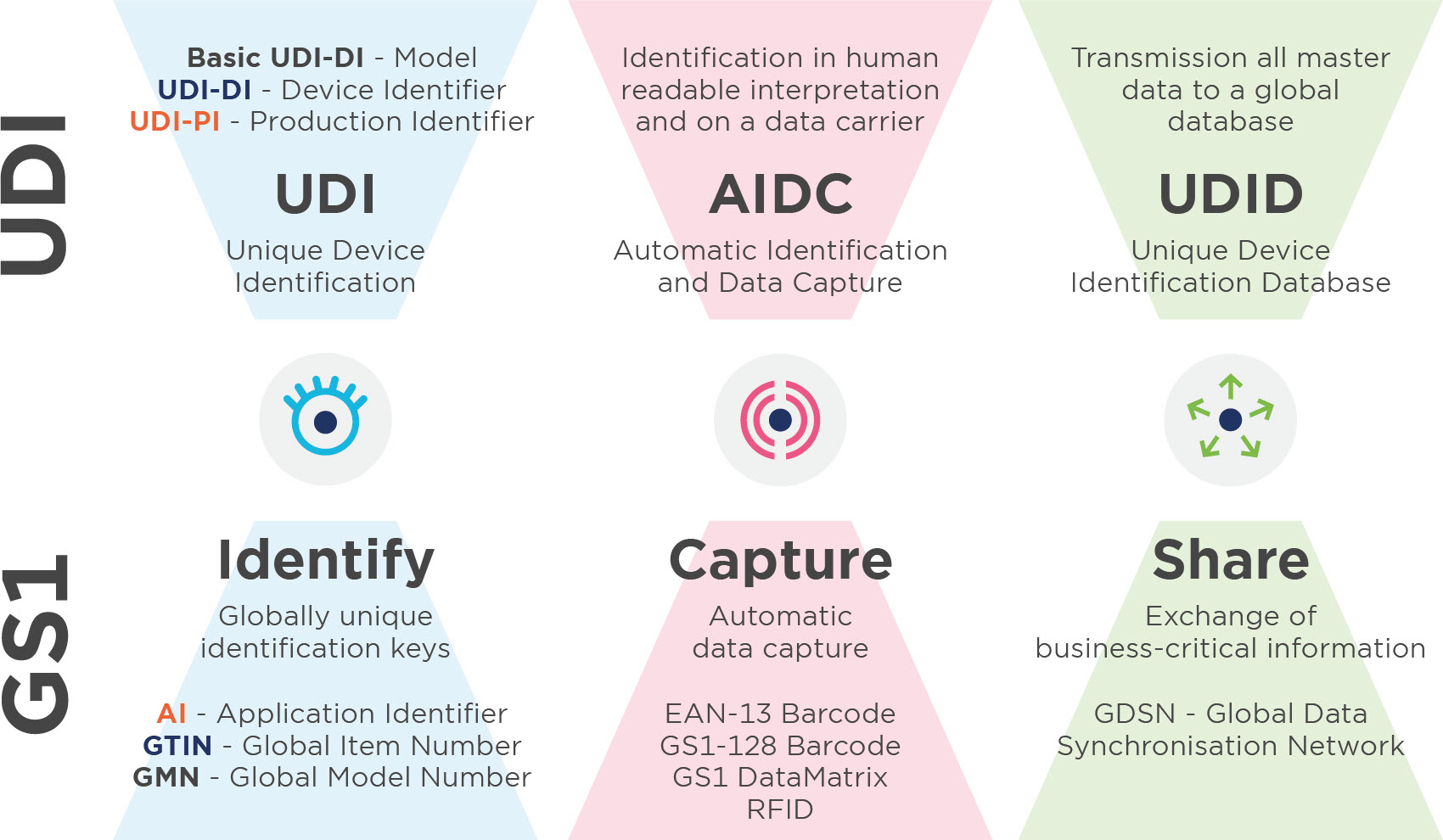
UDI - Unique Device Identification
UDI - Labelling of medical devices
Are you a manufacturer of medical devices (MD) and in-vitro diagnostics (IVD) and need to label your products in compliance with the UDI? GS1 Switzerland is an official UDI assignment centre.
Unique Device Identification (UDI) is a system for the identification, labelling and registration of medical devices. The aim is to increase patient safety through the complete traceability of products. UDI is currently legally binding for all manufacturers of medical devices and in-vitro diagnostics in Europe and the USA.
EU UDI
The Medical Device Regulation (MDR) and the In-Vitro Diagnostic Regulation (IVDR) have been in force since 2017. According to Swissmedic's legal basis, Switzerland has EU-equivalent medical device regulation.
Current updates on the MDR and IVDR are listed on the Swissmedic website, where you will also find an overview of the international standards used in Switzerland and the EUDAMED timeline.
Important note for the implementation of the UDI:
For Class I medical devices, it is mandatory to state the base UDI-DI on the EU Declaration of Conformity and the technical documentation.
We at GS1 Switzerland support you in the implementation of UDI.
Everything you need to know about the UDI-DI basis
5 steps to UDI
Where do I stand in the UDI implementation?
Before you start your work on UDI, you should consider the following 5 questions. You will realise that UDI is not an individual competition, but a group discipline that affects your entire value chain.
- Which products are affected by the MDR / IVDR?
- In which classes are these products categorised?
- Are there any changes to the classes?
- How many products are affected? - Do you already use the GS1 system?
- Is a GCP (Global Company Prefix) available?
- How is the GTIN allocation regulated? (GCP is required for GMN in EUDAMED / GUDID and/or GTIN) - Is broad UDI knowledge available in the company?
- Are the regulatory adjustments known?
- Are deadlines known? - How is UDI embedded in your company's organisation?
- Do you view UDI as a project or is it the responsibility of one person?
1. Gain GS1 Switzerland membership
To use GS1 standards, you need membership with use of the GS1 System (barcodes).
2. Assignement of a Basic UDI-DI
The MDR and IVDR define the Basic UDI-DI as the primary identifier of the device model, which is assigned at the level of the unit of use. It is the main key for records in the UDI database and is referenced in relevant certificates and EU declarations of conformity. Use the GMN – Global Model Number - for assignment of the Basic UDI-DI.
3. Assignement of UDIs
3.1. General requirements
The manufacturer assigns one-off UDIs for his medical devices and maintains these. The UDI may only be placed on the medical device or its packaging by the manufacturer. Each individual component or commercial trading product has its own separate UDI. Unless the components are part of a configurable product, which is labelled with its own UDI. Shipping containers or transport units are not affected by the UDI allocation, but are labelled with the Serial Shipping Container Code (SSCC), e.g. for shipment traceability.The GTIN Registry and MyGTIN help you to organise your GTINs.
3.2. The DI unit of use
The DI unit of use is used to assign the use of a medical device to a patient in cases where the UDI is not indicated on the individual medical device at the level of its unit of use, e.g. if several units of the same medical device are packaged together. To identify your units of use, use the GTIN – global trade item number.
3.3. UDI-DI
The UDI-DI is a one-time numeric code which is unique to a medical device model and is also used as 'access key' to information stored in a UDI database. The UDI-DI is unique at all levels of product packaging. To identify your medical devices and the available packaging levels, use one GTIN - global trade item number - each. Please note the relevant assignment and update rules (see MDR/IVDR Annex VI, Part C (3) and (6)) when assigning all identification numbers. These are enshrined in the MDR and IVDR on the one hand and on the other, there are GTIN assignment rules for healthcare.
3.4. UDI-PI
The UDI-PI is a numeric or alphanumeric code used to label the unit of device production. The different types of UDI-PI include serial number, lot number, software identification and the manufacturing or expiry date, or both. If a lot number, a serial number, software identification or an expiry date is indicated on the label, it is part of the UDI-PI. If the date of manufacture is also included on the labelling, this does not need to be incorporated in the UDI-PI. If only the date of manufacture is on the labelling, this must be used as UDI-PI. Use the GS1 application identifier (AI) for this purpose.
4. Selecting the appropriate UDI carrier
In principle, the requirements for UDI carriers listed in the MDR/IVDR must primarily be followed. They are provided in MDR/IVDR Annex VI Part C (4).
The following GS1 barcodes are permitted as UDI carriers:
-GS1-128
-GS1 DataMatrix
-EAN-13 Barcode ( expected until 2026)
RFID technology may also be used for the 1D or 2D barcodes. GS1 supports two frequency ranges – ultra high frequency (UHF) from 860 to 960 MHz and high frequency (HF) at 13.56 MHz.
5. Uploading UDI data to EUDAMED
You will need the Basic UDI-DI and all UDI-DIs generated as well as the associated medical device data prior to launch, in order to register your medical devices in the European database on medical devices (EUDAMED – see MDR/IVDR Annex VI Part A). You then continuously update this information. To date, no notification regarding the full functioning of EUDAMED has been published by the European Commission in the Official Journal of the European Union, which means that registration currently is not possible. However, you can already prepare data exchange as per the UDI regulations today.
GS1 Healthcare Videos
Important Links
The EU UDI Timeline
The medical devices legislation of the Federal Office of Public Health specifies which decisions of the EU Commission are applicable in Switzerland and when. In recent years, the EU has tightened the requirements for medical devices in order to improve the quality and safety of these products. The EU regulations on medical devices (MDR) and in vitro diagnostic medical devices (IVDR) came into force on 26 May 2017 and have been fully applicable since 26 May 2021 and 26 May 2022 respectively.
Switzerland has adapted its legislation in the same way in order to harmonise it with that of the EU. The provisions of the MDR have been incorporated into the TPA, the HRA, the MedDO and the new ClinO-Mep. The ordinances have been in force since 26 May 2021. The provisions of the IVDR were transposed into the TPA, the HRA, the new IvDO and the ClinO-Mep, which have been in force since 26 May 2022.
The agreement between the Swiss Confederation and the European Union on the mutual recognition of declarations of conformity (MRA) has stalled due to a lack of progress on institutional issues between Switzerland and the EU. The European Commission has so far refused to update this chapter. The Federal Office of Public Health provides ongoing information on the effects and the current status of development on its FOPH medical devices webpage.
UDI carrier on the labelling
A UDI carrier is affixed to the labelling of your products and to all higher packaging levels (with the exception of shipping containers) depending on the classification of the product in risk classes.
MD classes (see MDR Art. 123 para. 3f)
Class I

Class II

Class III

IVD classes (see IVDR Art. 113 para. 3e)
IVD Class D

IVD Class B & C

Class A

The deadlines for the introduction (arrows) of the risk classes are announced in the Swissmedic regulations. Please note that other deadlines apply for reusable devices for which the UDI carrier must be applied directly to the device.
Effects of the Medical Devices Ordinance (MedDO) in Switzerland
Due to a missing MRA (Mutual Recognition Agreement) between Switzerland and the EU, there are particularities for the Swiss market, which have to be considered as a manufacturer, importer or distributor of medical devices.
In Switzerland, the MedDo version of July 1, 2020 (as of May 26, 2021), which only partially adopts European law, has been in effect since May 26, 2021.
The missing MRA affects both imports and exports of medical devices. We focus our communication on imports. For exporting companies, the industry association SWISS MEDTECH has published detailed information.
The communication of the EU and also Switzerland repeatedly refer to the fact that talks are still ongoing and possible developments would be communicated. We will inform you as soon as possible about developments.
Conformity marking and identification number
(MedDO Art. 13 - Conformity marking and identification number)Devices placed on the market in Switzerland or made available on the Swiss market must bear a conformity marking in accordance with Annex 5. The conformity marking presented in Annex V to EU-MDR18 is also a permissible conformity marking.In addition to the CE mark described in Annex V of the EU MDR, Annex 5 of the MedDO also describes a national conformity mark. Both markings are permissible.
Product information
(MedDO Art. 16 - Product information)Product information must be written in all three official languages of Switzerland. Article 16 regulates in which cases the product information may be written in less than the three official languages or in English (paragraph 3).
Information on implantable devices
(MedDO Art. 20 - Information on implantable devices)For implantable products, the manufacturer must provide, in addition to the product information required under Article 16, the information required under Article 18 paragraph 1 EU-MDR33, including the implantation certificate. The implantation certificate must be written in all three official languages of Switzerland.
Registration of Economic Operators
(MedDO Art. 55 -Registration of Economic Operators)Manufacturers or their authorised representatives and importers must register the information required by part A of Annex VI to EU-MDR69 with Swissmedic within three months of placing a device on the market for the first time.Application form: Unique identification number according to Art- 55 MedDO (CHRN Swiss Single Registration Number).
Medical Devices Information System
(MedDO Section 2 - Medical Devices Information System)Articles 83 - 92 describe an information system for medical devices. Swissmedic is responsible for this information system and is responsible for drawing up regulations for its use. Data can be obtained from EUDAMED as well as from cantonal electronic systems.
Affixing the UDI
The deadlines for affixing the UDI, do not deviate from the EU MDR and are regulated as follows (MedDO Art. 104 - Affixing the UDI):
- for implantable and class III devices: from 26 May 2021;
- for class IIa and IIb devices: from 26 May 2023;
- for class I devices: from 26 May 2025;
- for reusable devices where the UDI has to be affixed to the product itself: two years after the dates given in letters a–c for the relevant product class.
Appointment of authorised representatives
(MedDO Art. 104a - Appointment of authorised representatives)If the manufacturer is domiciled in an EU or EEA state, or if the manufacturer has designated an authorised representative domiciled in an EU or EEA state, that manufacturer must designate an authorised representative in accordance with Article 51 paragraph 1 within the following time periods:
- for class III devices, class IIb implantable devices and active implantable devices: by 31 December 2021;
- for non-implantable class IIb devices and class IIa devices: by 31 March 2022;
- for class I devices: by 31 July 2022.
Proposed implementation of the Medical Devices Ordinance (MedDO) in Switzerland
We recommend three procedures for implementing the UDI so that you fulfil the requirements of the MDR/IVDR:
- Self-study
Our clearly organised website shows you how to implement the UDI in 5 steps. You will also find many helpful tutorials and further links that will be useful to you on the path to implementation. All you need is a membership with a number range to label your products. - Training and implementation
You attend one of our many UDI training courses to build up the knowledge you need. The knowledge we impart and the useful information on our website www.gs1.ch/udi will help you to implement the UDI. - Targeted training for your company
You don't have time to build up a UDI knowledge base and want to entrust us with this initial task? We help you to acquire targeted UDI knowledge by means of digital or on-site training courses. You choose the topics and the amount of time, we deliver the rest. Our UDI team will support you with the implementation. Get in touch with us and let us calculate your customised offer.
Labelling of medicinal products with the GS1 system
Unique identification of medicinal products
The GS1 system has been used in Switzerland for over 30 years for the identification and labeling of medicinal products. Until today almost 100 percent coverage of Swissmedic registered products has been achieved.
The identification and labeling of medicinal products across all package hierarchy are one of the cornerstones for the digitization of medication processes. They enable applications such as the federal law about the electronic patient record, the electronic vaccination card and the digitization of supply chain and clinical processes.
Identification as added value
The information is displayed by means of a barcode directly on the respective printed on the packaging level and are to all users along the supply chain and the clinical patient pathway available. Through the direct imprint of the identification keys and associated attributes on the packaging, lot of added value is generated. This information can be used at every station of the supply chain and the clinical patient pathway can be recorded directly and transferred to the IT systems.
Unambiguity increases safety
Due to the worldwide uniqueness of the identification keys, unlike proprietary identifications, errors are excluded. At the same time, patient safety is enhanced and supply chain processes made more efficient.
Agreement on uniform standard
In Switzerland, the stakeholders agreed 30 years ago to use the GS1 system and to identify medical products with a Global Trade Item Number (GTIN) In the meantime, most countries worldwide have decided to use the GS1 system for the identification of medicinal products .
Basics of medicinal products and labeling
Package hierarchy of medicinal products and their labeling
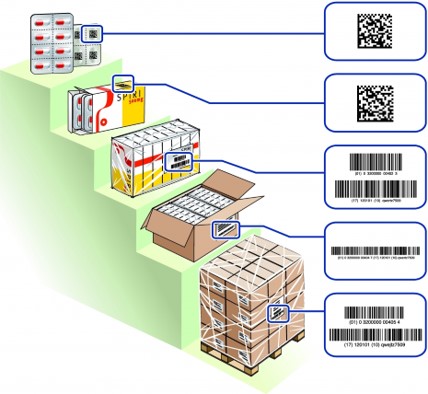
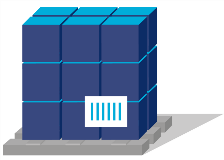
- Primary packaging
- Secondary packaging
- Tertiary packaging (e.g. bundle packaging)
- Quartenary packaging (e.g. carton and/or pallets)
| Package hierarchy | Product identification |
|
|
|---|---|---|---|
| Primary packaging | GTIN A, like 0 76 12345 00253 x |
GS1 DataMatrix | |
| Secondary packaging | GTIN B, like 0 7680 12345 678 x |
GS1 DataMatrix and/or EAN-13 | |
| Tertiary packaging (multipack/hospital pack) | GTIN C, like 0 76 12345 00254 x |
GS1-128 | |
| Quartenary packaging (carton and/or pallets) |
GTIN D, like 0 76 12345 00255 x |
GS1-128 |
Information on the labeling of primary packaging
Primary packaging is marked with a GS1 DataMatrix, which serves as a minimum information containing GTIN (product identification) and a lot number and ideally also the expiration date. Which information is contained in the GS1 DataMatrix depends on the space available for encryption. If possible, all information should be included in the barcode , but the data content has a direct impact on the barcode size. On the HRI (Human Readable Interpretation or plain writing line) can be waived for primary packaging if this is not possible for reasons of space. The award of the primary packaging is the basis for the digitization,the medication process and the bed side scanning process in the clinical patient pathway.
Information on the labeling of secondary packaging
Secondary packaging is marked with a GS1 DataMatrix, which contains the information GTIN (product identification), lot and expiry date contains. In the future, the GS1 DataMatrix of the secondary packaging will also include the unique serial number, which is used in the execution of the FMD (Falsified Medicine Directive). Besides, under or above the GS1 DataMatrix, there is an HRI (Human Readable Interpretation or plain writing line) to be attached, which the coded values. As there is no guarantee today that all POS systems in pharmacies and drugstores have 2-D Sacanners that are able to process GS1 DataMatrix Barcodes, an EAN-13 barcode can be printed on another packaging. This ensures that the barcode can be captured even with older POS systems. Labeling the packaging with a GS1 DataMatrix allows to add attributes to the barcode (Lot, EXP, Serial-Number) that can be read by the scanner and send them to the downstream IT systems. This enables advanced functions in POS systems, such as the control of the expiry date before of dispensation and the possibility of fully automatic storage with a robot.
Information on the labeling of tertiary packaging
Tertiary packaging is marked with a GS1-128 barcode containing the information GTIN (product identification), lot and expiry date. Next to, below or above the GS1-128 barcode, an HRI (Human Readable Interpretation or plain writing line) is to be placed, which shows the encoded values. For tertiary packages, a one-dimensional symbol is used because it is assumed that such packages could be stored and retrieved by an automated logistics system that cannot necessarily process a two-dimensional symbol.
Information on the labeling of quarternary packaging
Quaternary packaging is marked with a GS1-128 barcode containing the information GTIN (product identification), lot and expiry date. Next to, below or above the GS1-128 barcode, an HRI (Human Readable Interpretation or plain text line) is to be placed, which shows the encoded values. For quaternary packages, a one-dimensional symbol is used because it is assumed that such packages could be stored and retrieved by an automated logistics system that cannot necessarily process a two-dimensional symbol.
Barcodes for labeling medicinal products
For the labeling of medicinal products in Switzerland, in each case the three barcodes explained below can be used.
| Barcode | Data Content | Remarks |
| GTIN only | Can be read by 1D and 2D scanners Can be processed by all cash register systems with scanner in pharmacies. Application to secondary packaging |
|
|
(01)07612345002552 |
GTIN + attributes [GS1 Application Identifier Standard] |
Can only be read by 2D scanners Allows reading of information such as lot, EXP and serial number. Application to primary and secondary packaging |
| GTIN + attributes [GS1 Application Identifier Standard] |
Can be read by 1D and 2D scanners
|
What happens in the background when a barcode is read from a scanner?
Our explanatory film "Scanning a barcode" (Video in german) shows what action is triggered in an IT system after a barcode has been scanned. (Video in german)
Identification of medicinal products with Swissmedic authorisation
The GTIN is assigned exclusively for products manufactured by Swissmedic approved drugs and immunobiological products with 5-digit registration numbers and 3-digit package codes.
This data element has the following structure:
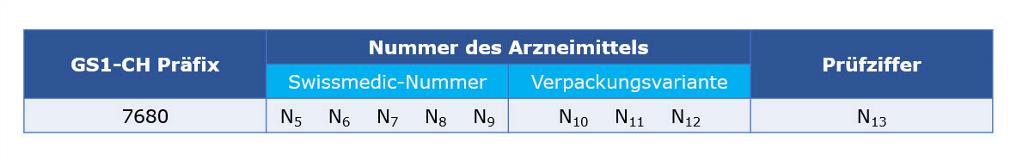
Notice:
All other medicinal products authorized by Swissmedic must be identified by a company's owned GTIN.
The marketing authorization holder is free to use this national solution for the identification and labeling of medicinal products or to use their own GTINs. This solution was introduced in 1984 established to improve drug safety and processes in Switzerland.
Medicinal products products approved in Switzerland are included for basic referencing in the refdatabase: www.refdata.ch
More Information:
Effects due to the implementation of the FMD in Switzerland
On the implementation of the Falsified Medicine Directive (FMD) in Switzerland: As part of the revision of the Therapeutic Products Act (TPA), a number of measures defined. The specifications were made by the European Union and the Swiss Federal Council and aim to protect patients against counterfeit medicines in the legal supply chain. The legal basis for this is the Anti-Counterfeiting Directive 2011/62/EU, the Delegated Regulation (EU) 2016/161 as well as Art. 17a TPA and the related ordinance, Art. 17a TPA.
These new regulatory requirements only affect RX drugs, i.e. Medicine products assigned to Swissmedic Lists A and B.
Note: Effects due to Corona
Due to the Corona pandemic, the FOPH is currently unable to issue any binding statement when the ordinance on Article Art. 17a TPA will enter into force. The FOPH has received the comments on the relevant consultation published. Download.
Individual recognition features in the supply chain
identification features and safety devices on the packaging of medicines. More information on the topic.
A pseudo-randomized Serial number serves as a recognition feature on each secondary packaging, which is printied by means of a GS1 DataMatrix barcode and an HRI (Human Readable Interpretation).
Parallel to this, a verification system is being set up in which the manufacturers upload their serial number information before the products come into the supply chain.
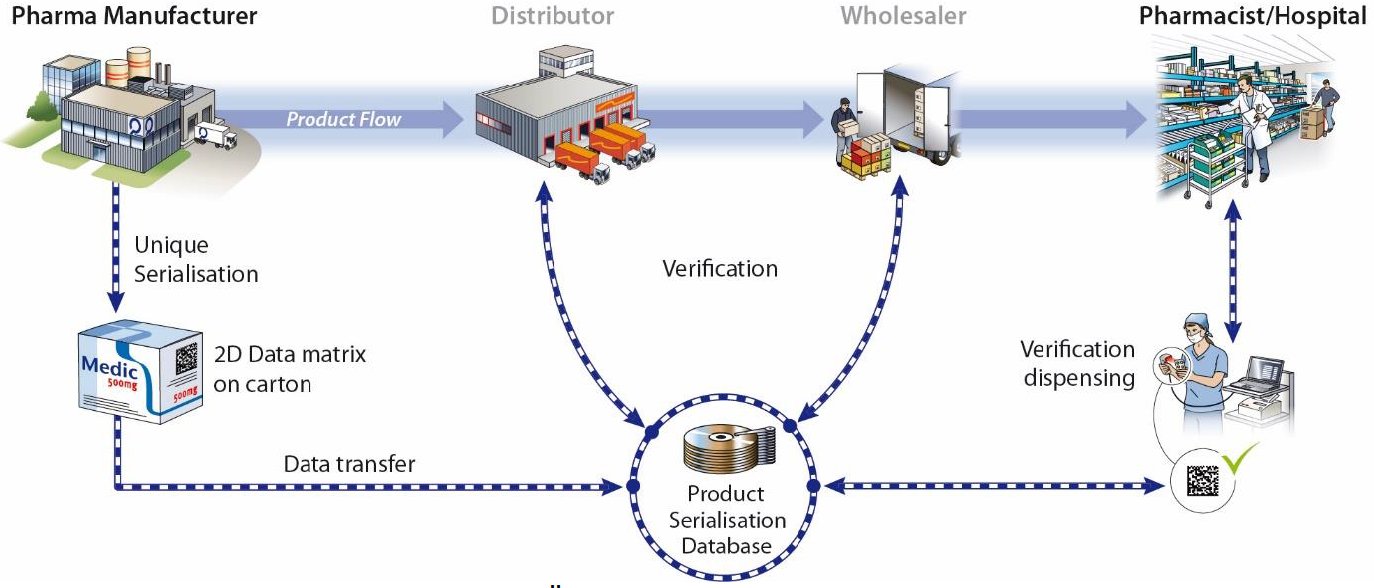
When dispensed at the pharmacy, hospital or doctor's office, the Data element on the package checked against the verification system and the respective package in the database as consumed marked. This system allows testing at all stations of the physical flow of goods and thus prevents the penetration of the neat supply chain with counterfeit products.
The safety devices on the packaging include in particular that in the future only packaging will be placed on the market be allowed to be "tamper proof" (first opening guarantee), i.e. a Packaging must be designed in such a way that it cannot be resealed.
The GS1 system allows medication and patient safety and simplifies the documentation in clinical information systems and increases the efficiency of the clinical patient pathway and the supply chain.

- The GS1 system is the basis for the digitization of the Health care.
- GS1 identification keys are used to identify pharmaceuticals, Patients, caregivers and locations clearly identified.
- With GS1 barcodes, identification and attributes are printed on every Object made available in machine and human readable form.
- With the master data exchange standard GDSN (Global Data Synchronization Network), master data can be shared across multiple parties and there is no need for time-consuming Mappings.
- The GS1 EDI standard simplifies the digitization of the Order-to-Cash Cycles.
Note
Do you need a pharmacode?
The Pharmacode can be obtained directly from HCI Solutions AG under the following coordinates: Koordinaten: www.hcisolutions.ch / Tel. 058 851 26 00
GTIN Registry
GTIN registration made easy
Create and manage your GTINs and EAN-13 barcodes easily now with the GTIN Registry, the free offer for our members.
How it works
Log in
Log in to the programme and create a new GTIN. The next free number is automatically displayed and the check digit is immediately calculated correctly.
Describe
Describe the product completely and correctly and upload a picture.
Create
Create a print-quality barcode with one click, download it and print it on the packaging.
Done
The product is now uniquely identified worldwide and ready for stationary trade and for sale at online marketplaces.
Frequently asked questions
What is a GTIN?
You need the GTIN, the "Global Trade Item Number", to create a barcode for the labelling of your products.
What is the GTIN Registry?
The GTIN Registry is an online tool. This tool allows you to create GS1 article numbers (GTINs) from the company prefix (GCP) and then download EAN-13 barcodes. In addition, you can enter product master data in structured form.
Who can use the GTIN Registry?
The GTIN Registry is a service for members of GS1 Switzerland who have licensed one or more company prefixes (GCP).
How do I get GTINs?
GTINs can be obtained by going to the ordering website: https://www.gs1.ch/en/barcodes-standards/barcodes
What are the benefits of using the GTIN Registry?
Thanks to the GTIN Registry:
- you always know which GTIN you have used for which product
- the next free GTIN is assigned directly to a new product
- there's no need to calculate the check digit
- you get the right barcode in the best quality and according to the requirements of the trade concerning valid and verified identification
Kann ich für meine MitarbeiterInnen einen Zugang einrichten?
he GTIN Registry is multi-user capable. This allows you to delegate GTIN management to your employees.
Make sure that your deputy has access to the GTIN Registry.
To do this, they must first create (register) an online account: To register.
Then let us know via the contact form for which persons (name, e-mail address) we may activate the access.
I have lost my password. What can I do?
Please use the "Forgotten password" function on www.gtinregistry.ch, which you will find directly under the login area. To do so, click on "Login" and on "Forgotten password" and follow the instructions step by step.