UDI - Labelling of medical devices
Are you a manufacturer of medical devices (MD) and in-vitro diagnostics (IVD) and need to label your products in compliance with the UDI? GS1 Switzerland is an official UDI assignment centre.
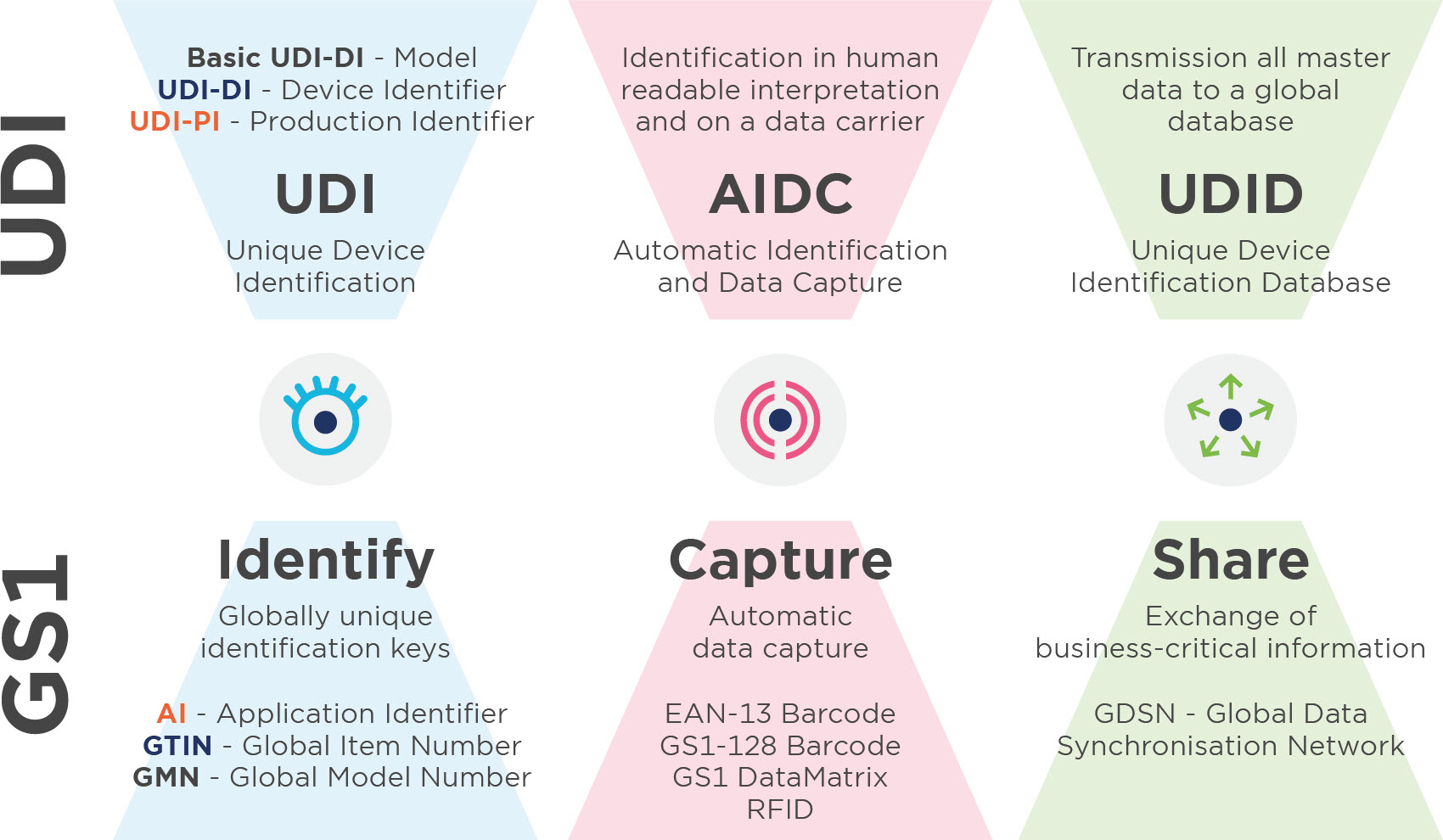
Unique Device Identification (UDI) is a system for the identification, labelling and registration of medical devices. The aim is to increase patient safety through the complete traceability of products. UDI is currently legally binding for all manufacturers of medical devices and in-vitro diagnostics in Europe and the USA.
EU UDI
The Medical Device Regulation (MDR) and the In-Vitro Diagnostic Regulation (IVDR) have been in force since 2017. According to Swissmedic's legal basis, Switzerland has EU-equivalent medical device regulation.
Current updates on the MDR and IVDR are listed on the Swissmedic website, where you will also find an overview of the international standards used in Switzerland and the EUDAMED timeline.
Important note for the implementation of the UDI:Important note for the implementation of the UDI:
For medical devices of the three classes I/IIa/IIb/III, the list of all assigned UDIs must be maintained for each medical device as part of the technical documentation. It is mandatory to state the base UDI-DI (GS1 = Global Model Number) on the EU Declaration of Conformity and the technical, clinical and safety documentation and certificates for registration and attestations. There are still transitional periods in some cases.
We at GS1 Switzerland support you in the implementation of UDI.
Everything you need to know about the UDI-DI basis
5 steps to UDI
Where do I stand in the UDI implementation?
Before you start your work on UDI, you should consider the following 5 questions. You will realise that UDI is not an individual competition, but a group discipline that affects your entire value chain.
- Which products are affected by the MDR / IVDR?
- In which classes are these products categorised?
- Are there any changes to the classes?
- How many products are affected? - Do you already use the GS1 system?
- Is a GCP (Global Company Prefix) available?
- How is the GTIN allocation regulated? (GCP is required for GMN in EUDAMED / GUDID and/or GTIN) - Is broad UDI knowledge available in the company?
- Are the regulatory adjustments known?
- Are deadlines known? - How is UDI embedded in your company's organisation?
- Do you view UDI as a project or is it the responsibility of one person?
1. Gain GS1 Switzerland membership
To use GS1 standards, you need membership with use of the GS1 System (barcodes).
2. Assignement of a Basic UDI-DI (only in Europe)
The UDI-DI base is the most important organisational feature for data records in the UDI database (MDR and IVDR) and is used to identify device models or device families. This appears in all documents (especially the declaration of conformity and technical documentation).
You can read more about this in the MedTech Europe document „Guidance on Basic UDI-DI Assignment“or in the UDI Guidance documents of the MDCG.
Use the Global Model Number (GMN) to create the basic UDI-DI. The Basic UDI_DI is never affixed to the product.
3. Assignement of UDIs
3.1. General requirements
The manufacturer assigns one-off UDIs for his medical devices and maintains these. The UDI may only be placed on the medical device or its packaging by the manufacturer. Each individual component or commercial trading product has its own separate UDI. Unless the components are part of a configurable product, which is labelled with its own UDI. Shipping containers or transport units are not affected by the UDI allocation, but are labelled with the Serial Shipping Container Code (SSCC), e.g. for shipment traceability.The GTIN Registry and MyGTIN help you to organise your GTINs.
3.2. The DI unit of use
The DI unit of use is used to assign the use of a medical device to a patient in cases where the UDI is not indicated on the individual medical device at the level of its unit of use, e.g. if several units of the same medical device are packaged together. To identify your units of use, use the GTIN – global trade item number.
3.3. UDI-DI (GS1:GTIN)
You assign a GTIN (= UDI-DI) to all items in your range. Not only utility units, but also higher packaging units should be assigned a GTIN (Global Trade Item Number). The UDI-DI is unique at all levels of product packaging.
Shipping units, customised products and test products are excluded.
The UDI-DI is a unique numeric code that is specific to a product model and also serves as an ‘access key’ to information in a UDI database.When assigning all identification numbers, please observe the respective allocation and amendment rules (see MDR/IVDR Annex VI, Part C, paragraphs 3 and 6). On the one hand, these are anchored in the MDR and IVDR on the one hand and on the other, there are GTIN allocation rules for healthcare sector.
3.4. UDI-PI (GS1: AI)
The UDI-PI is a numeric or alphanumeric code that is used to identify the production unit of the device. The most important UDI-PIs include the batch, serial and lot number, the expiry or manufacturing date and the software identification.If a batch number, serial number, software identification or expiry date is indicated on the labelling, this is part of the UDI-PI. If the date of manufacture is also on the labelling, this does not have to be included in the UDI-PI.If only the date of manufacture is on the labelling, this must be used as the UDI-PI. GS1 offers the GS1 application identifier (AI) for the assignment of UDI-PIs. The formats of the AIs must always be adhered to when creating UDI-PIs.
3.5 Master UDI-DI (MUDI)
There is a new Regulation (EU) 2017/745 regarding the assignment of unique product identifiers for contact lenses, spectacle lenses and finished reading devices. Highly individualised products with clear clinical similarities can be grouped together with a ‘Master UDI’ (MUDI). If you have any questions about the Master UDI-DI, please contact us.
4. Selecting the appropriate UDI carrier
In principle, the requirements for UDI carriers listed in the MDR/IVDR must primarily be taken into account. These can be found in MDR/IVDR Annex VI Part C Paragraph 4.
The following GS1 barcodes are permitted as UDI carriers:
-GS1-128
-GS1 DataMatrix
In addition to 1D or 2D barcodes, you can also use RFID technology. GS1 supports two frequency ranges - the ultra high frequency range (UHF) between 860 and 960 MHz, and the high frequency range (HF) at 13.56 MHz.
Do you want to be sure that the barcode you have created can be scanned? Then have it checked by the GS1 Switzerland barcode service. We check the print quality, data structure, layout and dimensions.
5. upload of UDI data (registration) in Europe and the USA
5a. Registration in regulatory databases SWISSDAMED (CH) and EUDAMED (EU)
You need the basic UDI-DI and all created UDI-DIs including the associated product data to register your products in the SWISSDAMED and EUDAMED databases for medical devices before placing them on the market.
You can then keep this information up to date at all times. You can prepare and enter the UDI device registration taking into account the EUDAMED functionality.
Remember that all processes for registering and updating UDI data must be documented in the QMS.
See also MDCG 2021-19.
Each basic UDI-DI (only in the EU) and also each UDI-DI is entered into the UDI database with an officially defined data set.
5b. Registration in the regulatory database GUDID (USA)
Medical devices that are supplied to the US market must be clearly identified and labelled in accordance with the UDI guidelines of the US FDA.
For manufacturers or ‘labellers’, this means that if you want to continue exporting your products to the US market, you must fulfil the UDI requirements.
Further details on the UDI requirements of the US FDA can be found on the FDA's very detailed website or on the GS1 US website. Please note that as a GS1 user you can fulfil the FDA requirements. An additional contract with GS1 US is generally not necessary.
The US UDI timeline
A detailed overview can be found here: Compliance Dates for UDI Requirements
Transmission of UDI data to GUDID (registration)
All information about the Global UDI Database (GUDID) can be found on the FDA website.
Detailed information
The EU UDI Timeline
The medical devices legislation of the Federal Office of Public Health specifies which decisions of the EU Commission are applicable in Switzerland and when. In recent years, the EU has tightened the requirements for medical devices in order to improve the quality and safety of these products. The EU regulations on medical devices (MDR) and in vitro diagnostic medical devices (IVDR) came into force on 26 May 2017 and have been fully applicable since 26 May 2021 and 26 May 2022 respectively.
Switzerland has adapted its legislation in the same way in order to harmonise it with that of the EU. The provisions of the MDR have been incorporated into the TPA, the HRA, the MedDO and the new ClinO-Mep. The ordinances have been in force since 26 May 2021. The provisions of the IVDR were transposed into the TPA, the HRA, the new IvDO and the ClinO-Mep, which have been in force since 26 May 2022.
The agreement between the Swiss Confederation and the European Union on the mutual recognition of declarations of conformity (MRA) has stalled due to a lack of progress on institutional issues between Switzerland and the EU. The European Commission has so far refused to update this chapter. The Federal Office of Public Health provides ongoing information on the effects and the current status of development on its FOPH medical devices webpage.
UDI carrier on the labelling
A UDI carrier is affixed to the labelling of your products and to all higher packaging levels (with the exception of shipping containers) depending on the classification of the product in risk classes.
MD classes (see MDR Art. 123 para. 3f)
Class I

Class II

Class III

IVD classes (see IVDR Art. 113 para. 3e)
IVD Class D

IVD Class B & C

Class A

The deadlines for the introduction (arrows) of the risk classes are announced in the Swissmedic regulations. Please note that other deadlines apply for reusable devices for which the UDI carrier must be applied directly to the device.
Effects of the Medical Devices Ordinance (MedDO) in Switzerland
Due to a missing MRA (Mutual Recognition Agreement) between Switzerland and the EU, there are particularities for the Swiss market, which have to be considered as a manufacturer, importer or distributor of medical devices.
In Switzerland, the MedDo version of July 1, 2020 (as of May 26, 2021), which only partially adopts European law, has been in effect since May 26, 2021.
The missing MRA affects both imports and exports of medical devices. We focus our communication on imports. For exporting companies, the industry association SWISS MEDTECH has published detailed information.
The communication of the EU and also Switzerland repeatedly refer to the fact that talks are still ongoing and possible developments would be communicated. We will inform you as soon as possible about developments.
Conformity marking and identification number
(MedDO Art. 13 - Conformity marking and identification number)Devices placed on the market in Switzerland or made available on the Swiss market must bear a conformity marking in accordance with Annex 5. The conformity marking presented in Annex V to EU-MDR18 is also a permissible conformity marking.In addition to the CE mark described in Annex V of the EU MDR, Annex 5 of the MedDO also describes a national conformity mark. Both markings are permissible.
Product information
(MedDO Art. 16 - Product information)Product information must be written in all three official languages of Switzerland. Article 16 regulates in which cases the product information may be written in less than the three official languages or in English (paragraph 3).
Information on implantable devices
(MedDO Art. 20 - Information on implantable devices)For implantable products, the manufacturer must provide, in addition to the product information required under Article 16, the information required under Article 18 paragraph 1 EU-MDR33, including the implantation certificate. The implantation certificate must be written in all three official languages of Switzerland.
Registration of Economic Operators
(MedDO Art. 55 -Registration of Economic Operators)Manufacturers or their authorised representatives and importers must register the information required by part A of Annex VI to EU-MDR69 with Swissmedic within three months of placing a device on the market for the first time.Application form: Unique identification number according to Art- 55 MedDO (CHRN Swiss Single Registration Number).
Medical Devices Information System
(MedDO Section 2 - Medical Devices Information System)Articles 83 - 92 describe an information system for medical devices. Swissmedic is responsible for this information system and is responsible for drawing up regulations for its use. Data can be obtained from EUDAMED as well as from cantonal electronic systems.
Affixing the UDI
The deadlines for affixing the UDI, do not deviate from the EU MDR and are regulated as follows (MedDO Art. 104 - Affixing the UDI):
- for implantable and class III devices: from 26 May 2021;
- for class IIa and IIb devices: from 26 May 2023;
- for class I devices: from 26 May 2025;
- for reusable devices where the UDI has to be affixed to the product itself: two years after the dates given in letters a–c for the relevant product class.
Appointment of authorised representatives
(MedDO Art. 104a - Appointment of authorised representatives)If the manufacturer is domiciled in an EU or EEA state, or if the manufacturer has designated an authorised representative domiciled in an EU or EEA state, that manufacturer must designate an authorised representative in accordance with Article 51 paragraph 1 within the following time periods:
- for class III devices, class IIb implantable devices and active implantable devices: by 31 December 2021;
- for non-implantable class IIb devices and class IIa devices: by 31 March 2022;
- for class I devices: by 31 July 2022.
We recommend three procedures for implementing the UDI so that you fulfil the requirements of the MDR/IVDR:
- Self-study
Our clearly organised website shows you how to implement the UDI in 5 steps. You will also find many helpful tutorials and further links that will be useful to you on the path to implementation. All you need is a membership with a number range to label your products. - Training and implementation
You attend one of our many UDI training courses to build up the knowledge you need. The knowledge we impart and the useful information on our website www.gs1.ch/udi will help you to implement the UDI. - Targeted training for your company
You don't have time to build up a UDI knowledge base and want to entrust us with this initial task? We help you to acquire targeted UDI knowledge by means of digital or on-site training courses. You choose the topics and the amount of time, we deliver the rest. Our UDI team will support you with the implementation. Get in touch with us and let us calculate your customised offer.
Proposed implementation of the Medical Devices Ordinance (MedDO) in Switzerland
Effects of the Medical Devices Ordinance (MedDO) in Switzerland
Due to a missing MRA (Mutual Recognition Agreement) between Switzerland and the EU, there are particularities for the Swiss market, which have to be considered as a manufacturer, importer or distributor of medical devices.
In Switzerland, the MedDo version of July 1, 2020 (as of May 26, 2021), which only partially adopts European law, has been in effect since May 26, 2021.
The missing MRA affects both imports and exports of medical devices. We focus our communication on imports. For exporting companies, the industry association SWISS MEDTECH has published detailed information.
The communication of the EU and also Switzerland repeatedly refer to the fact that talks are still ongoing and possible developments would be communicated. We will inform you as soon as possible about developments.
Conformity marking and identification number
(MedDO Art. 13 - Conformity marking and identification number)Devices placed on the market in Switzerland or made available on the Swiss market must bear a conformity marking in accordance with Annex 5. The conformity marking presented in Annex V to EU-MDR18 is also a permissible conformity marking.In addition to the CE mark described in Annex V of the EU MDR, Annex 5 of the MedDO also describes a national conformity mark. Both markings are permissible.
Product information
(MedDO Art. 16 - Product information)Product information must be written in all three official languages of Switzerland. Article 16 regulates in which cases the product information may be written in less than the three official languages or in English (paragraph 3).
Information on implantable devices
(MedDO Art. 20 - Information on implantable devices)For implantable products, the manufacturer must provide, in addition to the product information required under Article 16, the information required under Article 18 paragraph 1 EU-MDR33, including the implantation certificate. The implantation certificate must be written in all three official languages of Switzerland.
Registration of Economic Operators
(MedDO Art. 55 -Registration of Economic Operators)Manufacturers or their authorised representatives and importers must register the information required by part A of Annex VI to EU-MDR69 with Swissmedic within three months of placing a device on the market for the first time.Application form: Unique identification number according to Art- 55 MedDO (CHRN Swiss Single Registration Number).
Medical Devices Information System
(MedDO Section 2 - Medical Devices Information System)Articles 83 - 92 describe an information system for medical devices. Swissmedic is responsible for this information system and is responsible for drawing up regulations for its use. Data can be obtained from EUDAMED as well as from cantonal electronic systems.
Affixing the UDI
The deadlines for affixing the UDI, do not deviate from the EU MDR and are regulated as follows (MedDO Art. 104 - Affixing the UDI):
- for implantable and class III devices: from 26 May 2021;
- for class IIa and IIb devices: from 26 May 2023;
- for class I devices: from 26 May 2025;
- for reusable devices where the UDI has to be affixed to the product itself: two years after the dates given in letters a–c for the relevant product class.
Appointment of authorised representatives
(MedDO Art. 104a - Appointment of authorised representatives)If the manufacturer is domiciled in an EU or EEA state, or if the manufacturer has designated an authorised representative domiciled in an EU or EEA state, that manufacturer must designate an authorised representative in accordance with Article 51 paragraph 1 within the following time periods:
- for class III devices, class IIb implantable devices and active implantable devices: by 31 December 2021;
- for non-implantable class IIb devices and class IIa devices: by 31 March 2022;
- for class I devices: by 31 July 2022.
Proposed implementation of the Medical Devices Ordinance (MedDO) in Switzerland
GS1 Healthcare Videos
Important Links